To calculate the theoretical density of ceramics, five essential parameters are needed and these parameters are Number of formula units in unit cell (n’), Sum of atomic weights of atoms (ΣAc), Sum of atomic weights of anions (ΣAA), Unit cell volume (Vc) and Avogadro’s number (NA).
The formula for calculating theoretical density of ceramics:
ρ = n'(ΣAc + ΣAA) / VcNA
Where:
ρ = Theoretical Density of Ceramics
n’ = Number of Formula Units in Unit Cell
ΣAc = Sum of Atomic Weights of Atoms
ΣAA = Sum of Atomic Weights of Anions
Vc = Unit Cell Volume
NA = Avogadro’s Number
Let’s solve an example;
Find the theoretical density of ceramics when the number of formula units in unit cell is 12, the sum of atomic weights of atoms is 8, the sum of atomic weights of anions is 10, the unit cell volume is 9 and the avogadro’s number is 6.02214e+23.
This implies that;
n’ = Number of Formula Units in Unit Cell = 12
ΣAc = Sum of Atomic Weights of Atoms = 8
ΣAA = Sum of Atomic Weights of Anions = 10
Vc = Unit Cell Volume = 9
NA = Avogadro’s Number = 6.02214e+23
ρ = n'(ΣAc + ΣAA) / VcNA
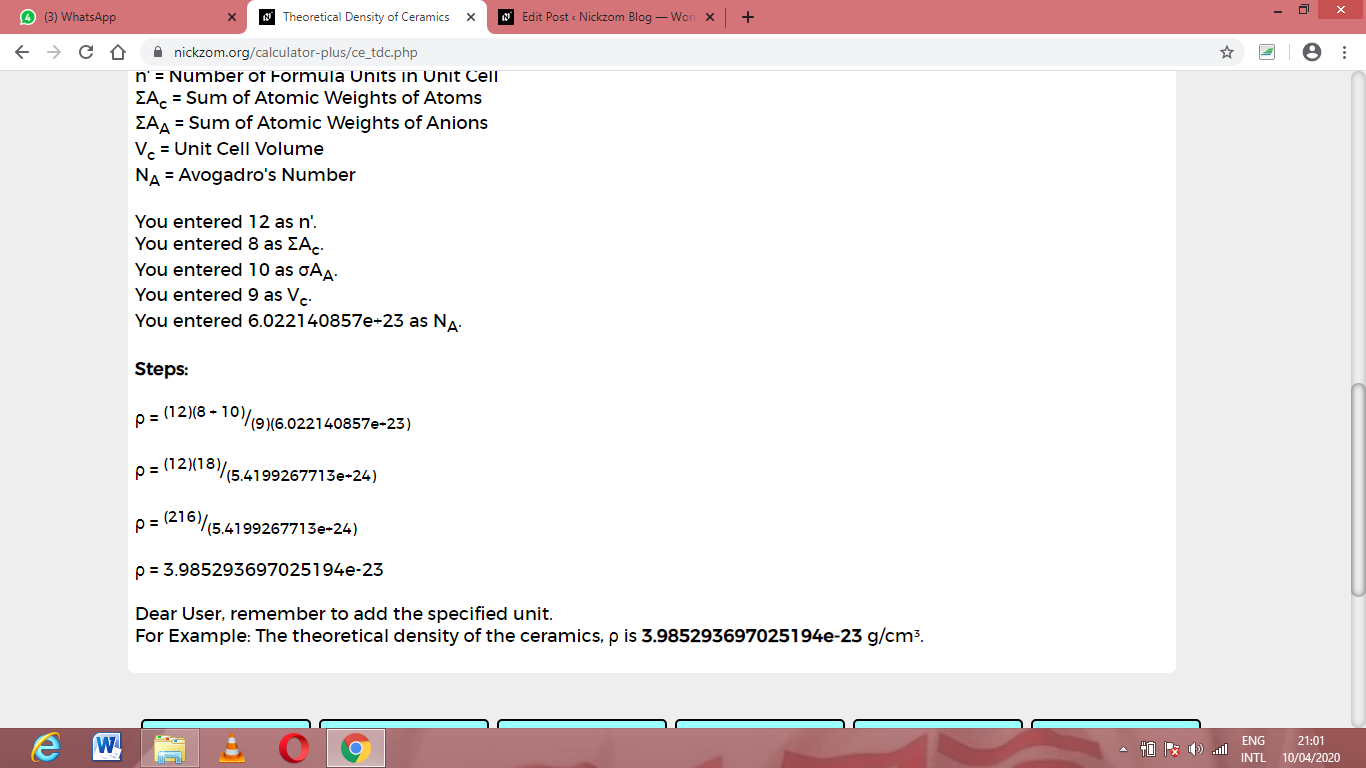
ρ = (12)(8 + 10) / (9)(6.02214e+23)
Then, ρ = (12)(18) / (5.4199e+24)
ρ = (216)/(5.4199e+24)
ρ = 3.98e-23
Therefore, the theoretical density of ceramics is 3.98e-23.
Calculating the Number of Formula Units in Unit Cell when the Theoretical Density of Ceramics, the Sum of Atomic Weights of Atoms, the Sum of Atomic Weights of Anions, the Unit Cell Volume and the Avogadro’s Number are Given
n’ = p x VcNA / (ΣAc + ΣAA)
Where;
n’ = Number of Formula Units in Unit Cell
ρ = Theoretical Density of Ceramics
ΣAc = Sum of Atomic Weights of Atoms
ΣAA = Sum of Atomic Weights of Anions
Vc = Unit Cell Volume
NA = Avogadro’s Number
Let’s solve an example;
Find the number of formula units in unit cell when the How to Calculate and Solve for Theoretical Density of Ceramics | Ceramics is 20, the sum of atomic weights of atoms is 12, the sum of atomic weights of anions is 10, the unit cell volume is 5 and the avogadro’s number is 6.022e+23.
This implies that;
ρ = Theoretical Density of Ceramics = 20
ΣAc = Sum of Atomic Weights of Atoms = 12
ΣAA = Sum of Atomic Weights of Anions = 10
Vc = Unit Cell Volume = 5
NA = Avogadro’s Number = 6.022e+23
n’ = p x VcNA / (ΣAc + ΣAA)
Then, n’ = 20 x 5 x 6.022e+23 / (12 + 10)
n’ = 6.022e+25 / (22)
n’ = 2737.72
Therefore, the number of formula units in unit cell is 2737.72.
Read more: How to Calculate and Solve for Flexural Strength for Rectangular Cross-section in Defects | Ceramics
Calculating the Sum of Atomic Weights of Atoms when the Theoretical Density of Ceramics, the Sum of Atomic Weights of Anions, the Number of Formula Units in Unit Cell, the Unit Cell Volume and the Avogadro’s Number are Given
ΣAc = p x VcNA / n’ – ΣAA
Where;
ΣAc = Sum of Atomic Weights of Atoms
ρ = Theoretical Density of Ceramics
n’ = Number of Formula Units in Unit Cell
ΣAA = Sum of Atomic Weights of Anions
Vc = Unit Cell Volume
NA = Avogadro’s Number
Let’s solve an example;
Find the sum of atomic weights of atoms when the theoretical density of ceramics is 24, the number of formula units in unit cell is 18, the sum of atomic weights of anions is 12, the unit cell volume is 8 and the avogadro’s number is 6.022e+23.
This implies that;
ρ = Theoretical Density of Ceramics = 24
n’ = Number of Formula Units in Unit Cell = 18
ΣAA = Sum of Atomic Weights of Anions = 12
Vc = Unit Cell Volume = 8
NA = Avogadro’s Number = 6.022e+23
ΣAc = p x VcNA / n’ – ΣAA
ΣAc = 24 x 8 x 6.022e+23 / 18 – 12
So, ΣAc = 1.156224e+23 / 18 – 12
ΣAc = 642346.6 – 12
ΣAc = 642334.6
Therefore, the sum of atomic weights of atoms is 642334.6.
Read more: How to Calculate and Solve for Normally Occupied Positions | Ceramics
Calculating the Sum of Atomic Weights of Anions when the Theoretical Density of Ceramics, the Sum of Atomic Weights of Atoms, the Number of Formula Units in Unit Cell, the Unit Cell Volume and the Avogadro’s Number are Given
ΣAA = p x VcNA / n’ – ΣAc
Where;
ΣAA = Sum of Atomic Weights of Anions
ρ = Theoretical Density of Ceramics
n’ = Number of Formula Units in Unit Cell
ΣAc = Sum of Atomic Weights of Atoms
Vc = Unit Cell Volume
NA = Avogadro’s Number
Master Calculations Instantly
Unlock solutions for math, physics, engineering, and chemistry problem with step-by-step clarity. No internet required. Just knowledge at your fingertips, anytime, anywhere.
Let’s solve an example;
Find the sum of atomic weights of anions when the theoretical density of ceramics is 32, the number of formula units in unit cell is 2, the sum of atomic weights of atoms is 6, unit cell volume is 10 and the avogadro’s number is 6.022e+23.
This implies that;
ρ = Theoretical Density of Ceramics = 32
n’ = Number of Formula Units in Unit Cell = 2
ΣAc = Sum of Atomic Weights of Atoms = 6
Vc = Unit Cell Volume = 10
NA = Avogadro’s Number = 6.022e+23
ΣAA = p x VcNA / n’ – ΣAc
ΣAA = 32 x 10 x 6.022e+23 / 2 – 6
That is, ΣAA = 1.92704e+25 / 2 – 6
ΣAA = 9.6352e-24 – 6
ΣAA = 3.6352e-24
Therefore, the sum of atomic weights of anions is 3.6352e-24.
Calculating the Unit Cell Volume when the Theoretical Density of Ceramics, the Sum of Atomic Weights of Atoms, the Sum of Atomic Weights of Anions, the Number of Formula Units in Unit Cell and the Avogadro’s Number are Given
Vc = n'(ΣAc + ΣAA) / p x NA
Where;
Vc = Unit Cell Volume
ρ = Theoretical Density of Ceramics
n’ = Number of Formula Units in Unit Cell
ΣAc = Sum of Atomic Weights of Atoms
ΣAA = Sum of Atomic Weights of Anions
NA = Avogadro’s Number
Let’s solve an example;
Find the unit cell volume when the theoretical density of ceramics is 10, the number of formula units in unit cell is 8, the sum of atomic weights of atoms is 3 and the sum of atomic weights of anions is 2.
This implies that;
ρ = Theoretical Density of Ceramics = 10
n’ = Number of Formula Units in Unit Cell = 8
ΣAc = Sum of Atomic Weights of Atoms =3
ΣAA = Sum of Atomic Weights of Anions = 2
NA = Avogadro’s Number = 6.022e+23
Vc = n'(ΣAc + ΣAA) / p x NA
Vc = 8 (3 + 2) / 10 x 6.022e+23
So, Vc = 8 (5) / 10 x 6.022e+23
Vc = 40 / 6.022e+24
Vc = 6.642e-23
Therefore, the unit cell volume is 6.642e-23.
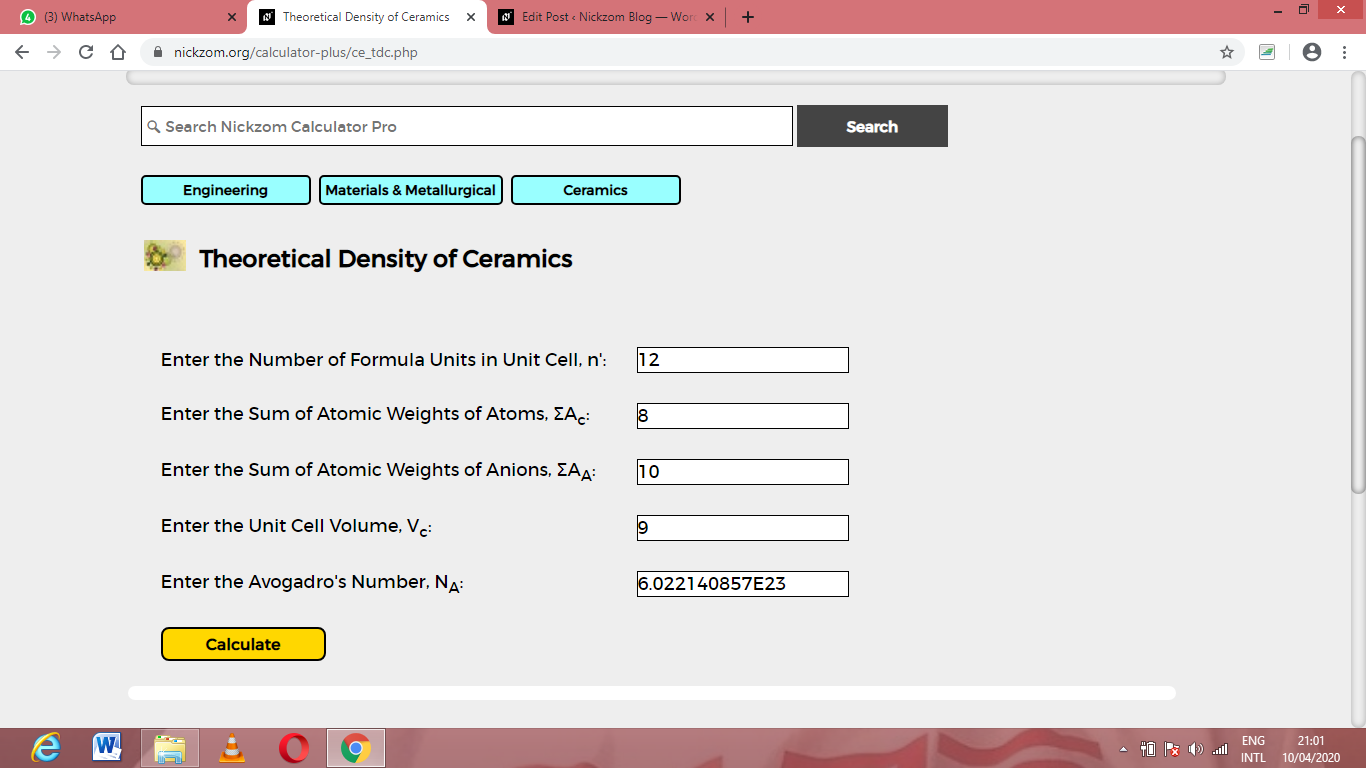
How to Calculate and Solve for Theoretical Density of Ceramics Using Nickzom Calculator
Nickzom Calculator – The Calculator Encyclopedia is capable of calculating the theoretical density of ceramics.
To get the answer and workings using the Nickzom Calculator – The Calculator Encyclopedia. First, you need to obtain the app.
You can get this app via any of these means:
Web – https://www.nickzom.org/calculator-plus
To get access to the professional version via web, you need to register and subscribe for NGN 1,500 per annum to have utter access to all functionalities.
You can also try the demo version via https://www.nickzom.org/calculator
Android (Paid) – https://play.google.com/store/apps/details?id=org.nickzom.nickzomcalculator
Android (Free) – https://play.google.com/store/apps/details?id=com.nickzom.nickzomcalculator
Apple (Paid) – https://itunes.apple.com/us/app/nickzom-calculator/id1331162702?mt=8
Once, you have obtained the calculator encyclopedia app, proceed to the Calculator Map, then click on Materials & Metallurgical under Engineering.
Now, Click on Ceramics under Material & Metallurgical
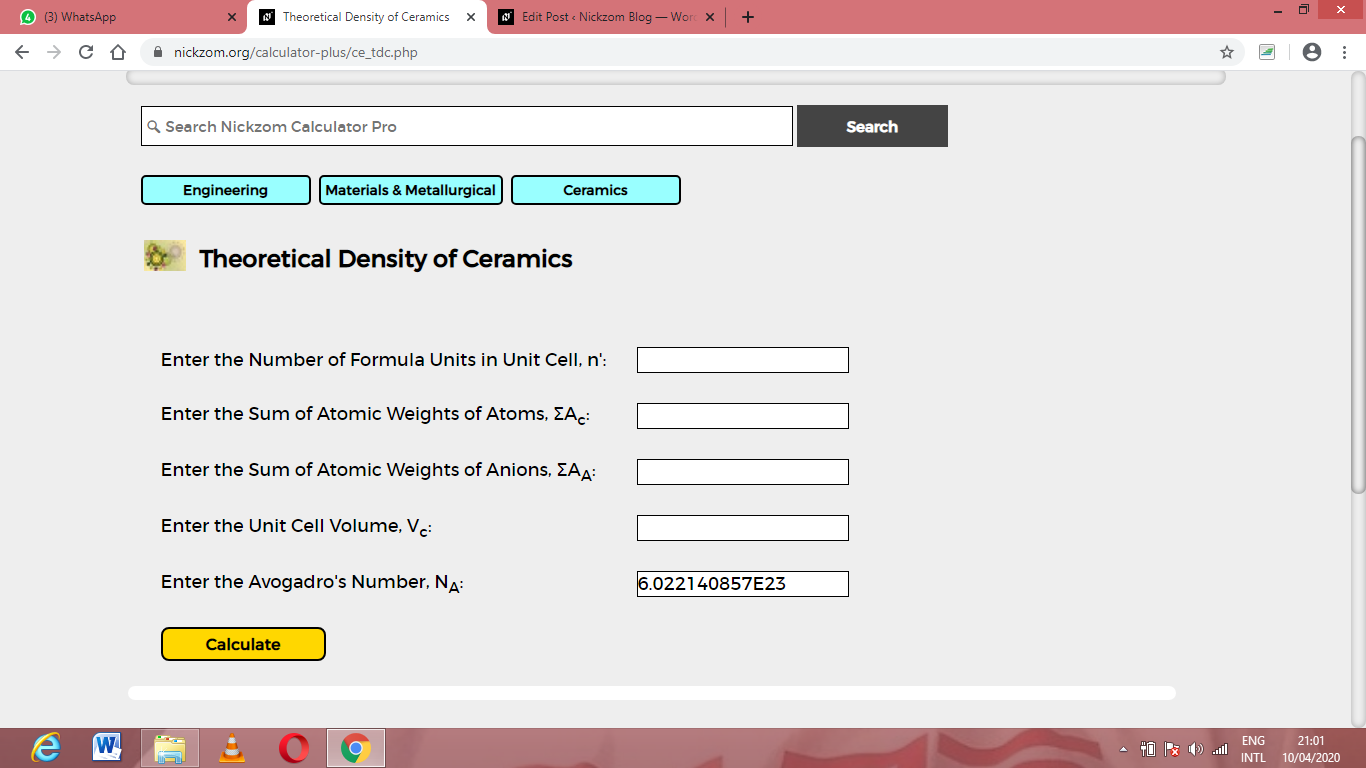
Now, Click on Theoretical Density of Ceramics under ceramics
The screenshot below displays the page or activity to enter your values, to get the answer according to the respective parameters which are the Number of formula units in unit cell (n’), Sum of atomic weights of atoms (ΣAc), Sum of atomic weights of anions (ΣAA), Unit cell volume (Vc) and Avogadro’s number (NA).
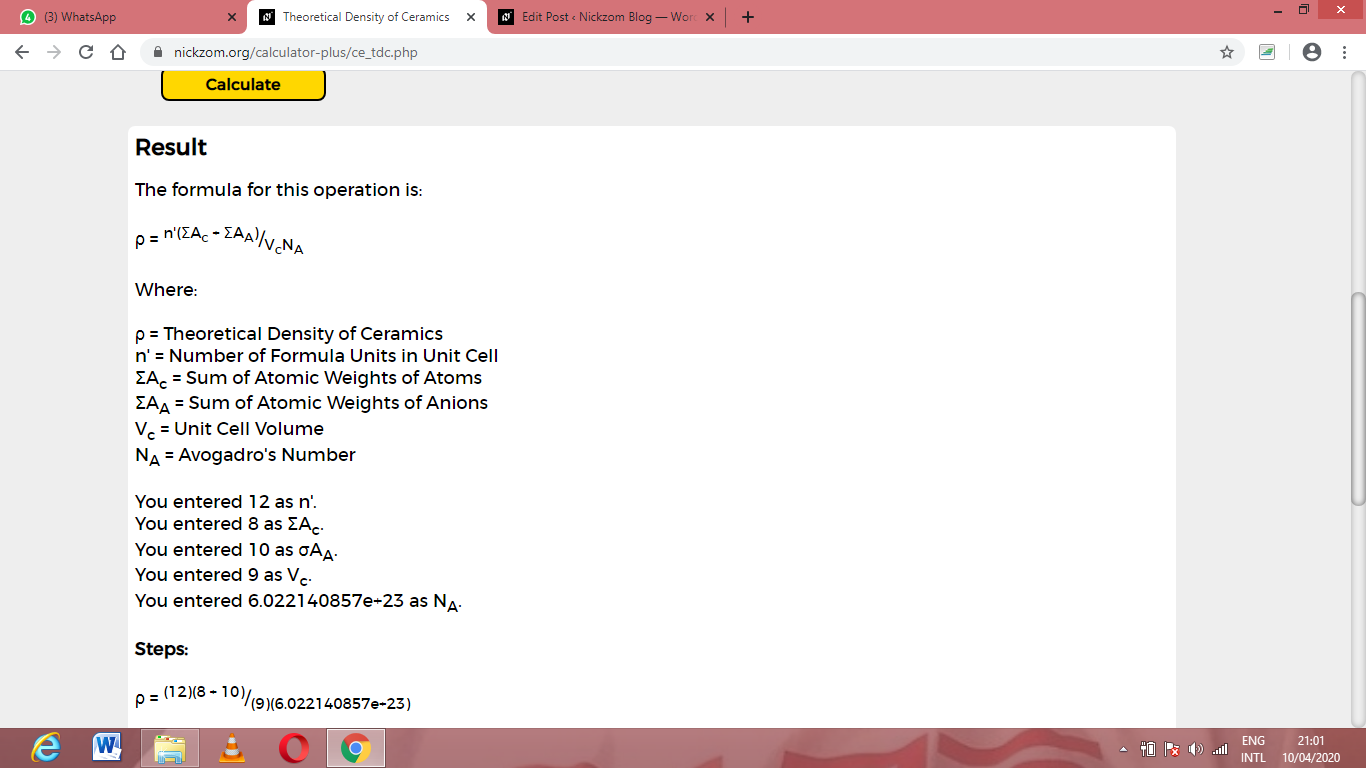
Now, enter the values appropriately and accordingly for the parameters as required by the Number of formula units in unit cell (n’) is 12, Sum of atomic weights of atoms (ΣAc) is 8, Sum of atomic weights of anions (ΣAA) is 10, Unit cell volume (Vc) is 9 and Avogadro’s number (NA) is 6.022e+23.
Finally, Click on Calculate
Master Calculations Instantly
Unlock solutions for math, physics, engineering, and chemistry problem with step-by-step clarity. No internet required. Just knowledge at your fingertips, anytime, anywhere.
As you can see from the screenshot above, Nickzom Calculator– The Calculator Encyclopedia solves for the theoretical density of ceramics and presents the formula, workings and steps too.