The image above represents rate of desorption. To calculate rate of desorption, two essential parameters are needed and these parameters are Desorption Proportionality Constant (Kd) and Fraction of Surface Covered by Adsorbed (θ).
The formula for calculating rate of desorption:
R = Kdθ
Where:
R = Rate of Desorption
Kd = Desorption Proportionality Constant
θ = Fraction of Surface Covered by Adsorbed
Let’s solve an example;
Given that the desorption proportionality constant is 10 and the fraction of surface covered by adsorbed is 12. Find the rate of desorption?
This implies that;
Kd = Desorption Proportionality Constant = 10
θ = Fraction of Surface Covered by Adsorbed = 12
That is, R = Kdθ
R = (10)(12)
R = 120
Therefore, the rate of desorption is 120.
Calculating the Desorption Proportionality Constant when the Rate of Desorption and the Fraction of Surface Covered by Adsorbed are Given
Kd = R / θ
Where:
Kd = Desorption Proportionality Constant
R = Rate of Desorption
θ = Fraction of Surface Covered by Adsorbed
Let’s solve an example;
Find the desorption proportionality constant when the rate of desorption is 20 and the fraction of surface covered by adsorbed is 4.
This implies that;
R = Rate of Desorption = 20
θ = Fraction of Surface Covered by Adsorbed = 4
Kd = R / θ
That is, Kd = 20 / 4
Kd = 5
Therefore, the desorption proportionality constant is 5.
Read more: How to Calculate and Solve for Rate of Absorption | Mineral Processing
Calculating the Fraction of Surface Covered by Adsorbed when the Rate of Desorption and the Desorption Proportionality Constant are Given
θ = R / Kd
Where:
θ = Fraction of Surface Covered by Adsorbed
R = Rate of Desorption
Kd = Desorption Proportionality Constant
Let’s solve an example;
Find the fraction of surface covered by adsorbed when the rate of desorption is 12 and the desorption proportionality constant is 3.
This implies that;
R = Rate of Desorption = 12
Kd = Desorption Proportionality Constant = 3
θ = R / Kd
Then, θ = 12 / 3
θ = 4
Therefore, the desorption proportionality constant is 4.
Read more: How to Calculate and Solve for Rate Constant | Mineral Processing
How to Calculate Rate of Desorption With Nickzom Calculator
Nickzom Calculator – The Calculator Encyclopedia is capable of calculating the rate of desorption.
To get the answer and workings of the rate of desorption using the Nickzom Calculator – The Calculator Encyclopedia. First, you need to obtain the app.
Master Calculations Instantly
Unlock solutions for math, physics, engineering, and chemistry problem with step-by-step clarity. No internet required. Just knowledge at your fingertips, anytime, anywhere.
You can get this app via any of these means:
Web – https://www.nickzom.org/calculator-plus
To get access to the professional version via web, you need to register and subscribe to have utter access to all functionalities.
You can also try the demo version via https://www.nickzom.org/calculator
Android (Paid) – https://play.google.com/store/apps/details?id=org.nickzom.nickzomcalculator
Android (Free) – https://play.google.com/store/apps/details?id=com.nickzom.nickzomcalculator
Apple (Paid) – https://itunes.apple.com/us/app/nickzom-calculator/id1331162702?mt=8
Once, you have obtained the calculator encyclopedia app, proceed to the Calculator Map, then click on Materials and Metallurgical under Engineering.
Now, Click on Mineral Processing under Materials and Metallurgical
Now, Click on Rate of Desorption under Mineral Processing
The screenshot below displays the page or activity to enter your values, to get the answer for the rate of desorption according to the respective parameter which is the Desorption Proportionality Constant (Kd) and Fraction of Surface Covered by Adsorbed (θ).
Now, enter the values appropriately and accordingly for the parameters as required by the Desorption Proportionality Constant (Kd) is 14 and Fraction of Surface Covered by Adsorbed (θ) is 24.
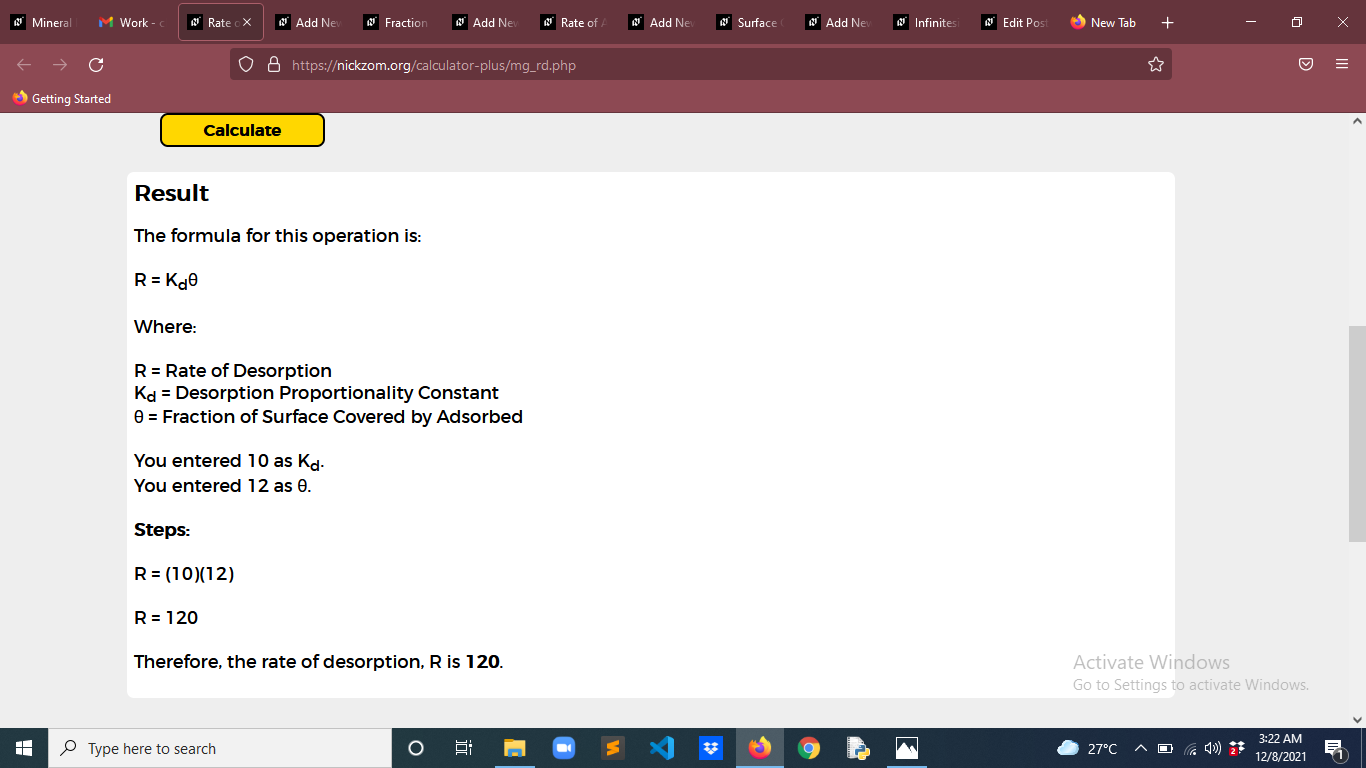
Finally, Click on Calculate
As you can see from the screenshot above, Nickzom Calculator– The Calculator Encyclopedia solves for the rate of desorption and presents the formula, workings and steps too.